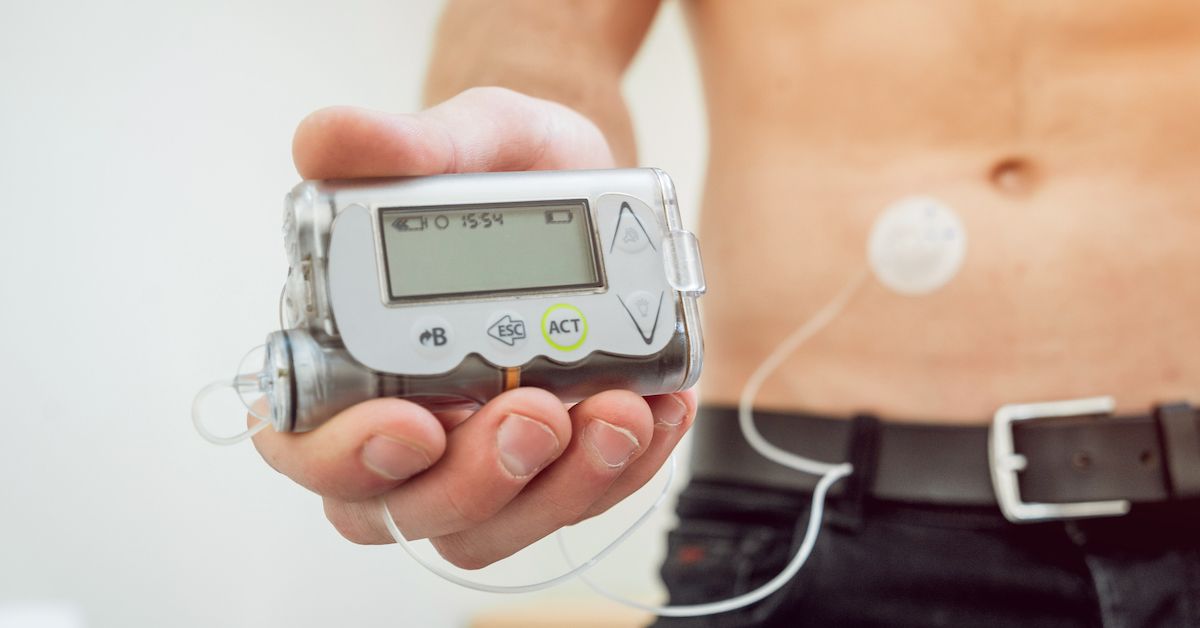
Last week the U.S. Food & Drug Administration (FDA) announced a massive recall on insulin pumps manufactured by Medtronic. The FDA identifies this as a Class I recall, which means that “use of these devices may cause serious injuries or death.”
The devices included in the recall are for patients with type 1 diabetes who are:
The affected devices have missing or broken retainer rings, which prevents insulin cartridges from locking into place. If the cartridge is loose, the wrong dosage of insulin may be delivered.
One person has died and over 2,000 injuries have been reported from defects with these MiniMed insulin pumps. More than 322,000 insulin pump system devices are included in the MiniMed recall.
Medtronic is advising customers with defective pumps to contact the manufacturer for a replacement device. However, if you have suffered injury or lost a loved one due to defective MiniMed insulin pumps, you may be eligible for compensation through a product liability case.

If you have type 1 diabetes and use one of the MiniMed insulin pump systems, your first step is to check the device to see if the retainer ring is damaged or missing. The insulin cartridge will feel loose if the retainer ring is not intact.
If you are the parent of a child with type 1 diabetes who uses a MiniMed device, help him or her check the device for defects. In the event that your child drops the pump, be sure to check the retainer ring and insulin cartridge to ensure it didn’t sustain damage that may affect the dosages delivered by the device.
According to the recall notice, if your device is intact, you can continue to use it as normal. If, however, the ring is broken or missing from your device or the insulin cartridge does not fit properly, you should contact Medtronic to replace the pump. In the meantime, talk to your doctor about switching from the pump system to manual injections of insulin.
Patients with diabetes place enormous trust in devices like the MiniMed Insulin Pump System to help them manage their blood sugar levels. Insulin pumps are an attractive alternative to injecting yourself with insulin multiple times every day. The pump systems can be programmed to deliver varying levels of insulin at different times of day. Doses can also be manually delivered with the push of a button.
Many patients find the portability and programmability of insulin pumps attractive. Unfortunately, when these devices fail, the outcomes can be catastrophic.
Insulin dosing is thrown off when the cartridges are not securely fastened in place. Diabetics who get too much insulin may develop hypoglycemia, or low levels of blood sugar. Symptoms of hypoglycemia include:
Patients with untreated hypoglycemia may suffer seizures and loss of consciousness. In extreme cases, chronically low blood sugar can be fatal.
Damage to or loss of the retainer ring on an insulin pump can also result in underdosing of insulin. Diabetics already suffer from hyperglycemia, or elevated blood sugar levels, so insufficient doses of insulin can result in symptoms such as:
Patients with untreated hyperglycemia may have difficulty breathing or suffer from nausea, vomiting, diarrhea, and fever. They might even exhibit signs of confusion and, in severe instances, slip into a coma. Diabetic coma is a life-threatening complication of diabetes.
Better care and advances in technology have improved patients’ ability to manage their own diabetes. Unfortunately, when the devices that make self-care possible malfunction or are subject to defects, the risk of serious injury or death increases.
If you discover a defect with your MiniMed insulin pump device, you and your doctor should discuss whether or not you should continue insulin pump treatment or opt for injection therapy. Should you decide to continue using an insulin pump system, contact Medtronic to inquire about a replacement device.
Under NO circumstances should you throw away the defective insulin pump. If you suffered adverse effects due to over- or underdosing of insulin as a result of the defect, you will need to hang on to the device to prove that your pump system was 1). defective and 2). part of this recall.
The same is true if your loved one suffered serious injury or tragically died because of an insulin pump defect. It may be an unpleasant reminder of what you and your family have been through, but one of the keys in any defective product case is proving that you were impacted by the dangerous product.
Fortunately, storing an insulin pump when it’s not in use should be very simple. The device is only about the size of a tape recorder, so it will easily fit in a disused cupboard or closet. If you still have the original packaging, it is even better to store the pump in that. Not only does the package safely store the pump, but it is additional proof that your device is among those included in the recall because of the faulty design.
Patients with type 1 diabetes must spend a significant portion of their entire lives managing blood sugar levels. Insulin pumps are designed to make managing your diabetes easier. However, defects in MiniMed devices by Medtronic can lead to severe injury and even death.
Attorneys at Dreyer Boyajian LLP are currently investigating defective insulin pump cases. If you were injured or your loved one passed away due to complications of unmanaged blood sugar associated with defective MiniMed 630G or MiniMed 670G systems, our law firm has the experience and resources to provide the assistance you need.
Please contact our law offices in Albany or Sarasota Springs for a free evaluation of your claim. Dreyer Boyajian LLP has been serving clients in New York state for over 30 years in cases involving catastrophic injury and death resulting from defective products.
Notifications